-
PDF
- Split View
-
Views
-
Cite
Cite
Vibha Bhatnagar, Lin Liu, Caroline M. Nievergelt, Erin Richard, Victoria H. Brophy, Braj Pandey, Michael S. Lipkowitz, Daniel T. O'Connor, Paraoxonase 1 (PON1) C/T-108 Association With Longitudinal Mean Arterial Blood Pressure, American Journal of Hypertension, Volume 25, Issue 11, November 2012, Pages 1188–1194, https://doi.org/10.1038/ajh.2012.106
Close - Share Icon Share
Abstract
Blood pressure is a complex quantitative trait with a strong genetic component. In this study, we leveraged the Veterans Affairs electronic medical record system to explore the relationship between Paraoxonase 1 (PON1)-108 C/T (rs705379) and mean arterial blood pressure (MAP).
Outpatient blood pressure data over an approximate 8-year period was collected from the Veterans Affairs Hypertensive Cohort (N = 1,302). Association between genotype and longitudinal MAP was further explored using a random effects model controlling for age, ancestry, renal function, and other determinants of blood pressure. To control for population stratification, principal component groupings based on ancestry informative markers in this dataset were included as covariates (in addition to self-identified ancestry). Data from the African American Study of Kidney Disease and Hypertension (AASK, N = 857) was used to confirm significant findings in an independent cohort.
There was a significant interaction between PON1-108 C/T genotype and follow-up age group. At a younger age (<50 years), there was an estimated 2.53 mm Hg (95% confidence interval: 1.06, 4.00) increase in MAP with each additional C-allele. At the older age groups, there were no significant associations between PON1-108 C/T genotype and MAP. Using data from the AASK trial, the C-allele at PON1-108 C/T was significantly associated with a higher MAP (P = 0.005) but only among younger participants (<54 years).
The PON1-108 polymorphism may be associated with MAP in an age-dependent manner.
Hypertension is one of the most important risk factors for cardiovascular disease and renal failure in the United States. Blood pressure is a complex quantitative trait and varies with activity, lifestyle (salt intake, exercise, alcohol consumption etc.), comorbid medical disease, stress levels, and age. Although environmental and lifestyle factors play a crucial role in modulating the hypertensive phenotype, heritability estimates of hypertension are moderate to high, ranging from 20 to 55%.1–3 However, the contribution of a single genetic marker is likely to be limited because of the involvement of multiple gene variants, epigenetic interactions between genes and the environment and interactions between genes in several physiological pathways.
Paraoxonase 1 (PON1) is a serine esterase secreted by the liver, originally identified because of its role in the detoxification of paraoxon and other organophosphates.4 It is located on chromosome 7 and is a member of a gene family that includes PON2 and PON3.5PON1 appears to be a significant physiological mediator of oxidative stress, modulating the release of pro-inflammatory enzymes resulting in atherosclerosis and subsequent plaque formation. It is associated with high-density lipoprotein and prevents oxidation of low-density lipoprotein.6 Homocysteine thiolactone, a highly reactive metabolite of homocysteine, is another endogenous substrate for PON1; some of the cardio-protective effects of PON1 may be partially mediated through homocysteine thiolactone clearance.7 There is strong linkage disequilibrium across the PON1 gene, and PON1 activity has been shown to be up-regulated by the C-allele at -108 (rs705379).8 However, PON1 activity is also heavily modulated by environmental factors with much individual variation independent of genotype.9,10
Because of the association with atherosclerosis, we hypothesized that PON1 variation may be associated with blood pressure. In this study, we leveraged the extensive Veterans Affairs (VA) electronic medical record system to systematically phenotype and collect outpatient blood pressure data over an average of 8 years from a multi-ethnic VA Hypertensive Cohort (VAHC)11. The association between PON1-108 C/T genotypes and mean arterial blood pressure (MAP), a composite of systolic and diastolic blood pressure, was then modeled at follow-up age groups (<50 years, 50–70 years, and >70 years) controlling for determinants of hypertension.
Methods
Study population. Details of the multi-ethnic VAHC have been previously published.11 Protocol approval and informed consent were obtained according to the requirements of the University of California San Diego Human Subjects Protection Program and the Research Committee of the San Diego VA Medical Center. Briefly, 1,527 participants from the VA San Diego Healthcare System were recruited to participate in a long-term follow-up study. Of these, 1,302 (89%) with phenotype and genetic data were in included in this analysis.
The majority were men (96%) with an average age of 64.3 ± 12.7 years. As described in our previous work, participants were recruited either in clinic or by mail; clinic recruits were younger with higher blood pressures. Data was extracted from the regional Veterans Integrated System Network 22 from 1999 to December 2007; data included detailed biomedical parameters (labs, vital signs, body mass index etc.) and medical diagnoses. Study participants had an average of 87.4 ± 22.9 months of follow-up per person and 47 (range: 1–582) outpatient blood pressure readings per person. In addition to using the electronic medical records, study participants completed a survey designed to query lifestyle parameters such as salt intake, tobacco, and alcohol intake.
Ancestry and population stratification. Participants were self-identified as European American (N = 876), African American (N = 204), Asian American (N = 49), Hispanic/Latino (N = 78), Native American (N = 27), or unidentified (N = 68). The main analysis focused on European and African Americans. Since there were few members in the other racial groupings, the remaining groups were collapsed, limiting the ability to interpret the results for this third group. In addition to self- identified ethnicity, 87 autosomal ancestry informative markers from our data set were used in a multi-dimensional scaling analysis using the PLINK routine (http://pngu.mgh.harvard.edu/purcell/plink/),12 and the first four multi-dimensional scaling components were included as covariates to adjust for residual population stratification.
Genetics. As previously detailed, buccal cell DNA was collected at the time of recruitment and stored; 1,474 (~96.5%) buccal cell samples yielded good quality (polymerase chain reaction-amplifiable) DNA, with an average DNA yield of 5.02 ± 0.12µg, by PicoGreen fluorescence (Invitrogen, Carlsbad, CA).11 In this study, we focused on a promoter region polymorphism, -108 C/T on PON1.
Data analysis
Bivariate analysis. The bivariate associations between average MAP and other potential determinants of blood pressure were first explored. Associations between PON1-108 C/T genotypes and determinants of blood pressure were also explored. These determinants included other biomedical parameters such as serum creatinine, cholesterol and glycosolated hemoglobin A1C used to measure glucose control in patients with diabetes, medical diagnoses including type 2 diabetes, renal disease, cardiovascular disease, cardiac arrhythmias (including atrial and ventricular arrhythmias) and cerebrovascular disease, Charlson score (a composite measure of 17 comorbidities),13,14 and number of antihypertensive medications at recruitment. Lifestyle characteristics included perceived stress levels, salt intake, alcohol, and tobacco use.
Bivariate associations were tested using the Kruskal–Wallis test for continuous variables, and the χ2 test or Fisher's exact test for categorical variables. Variables that were associated with either average MAP or genotype (P < 0.15) were considered as covariates in the longitudinal modeling analysis described below.
Longitudinal modeling of MAP. The association between PON1-108 C/T genotypes and longitudinal MAP (over an approximate 8 years per study participant) was further explored using a random effects model. Follow-up age was categorized as younger adult (<50 years), middle-aged adult (50–70 years) and older adult (>70 years). An a priori model was not specified for genotypes; continuous/additive, categorical, and dichotomous (dominant/recessive) models were considered. In addition, a genotype by follow-up age interaction was also included in the base model. Random intercept and random age effect were considered and the significance of random effect was assessed using the likelihood ratio test.
The association between each potential covariate and blood pressure was also assessed using a random effects model. If a variable was significantly associated (P value <0.15) with blood pressure in the random effects model and also significantly associated with genotype in the bivariate association analysis described above, the variable was included as a covariate in the multivariable random effects model. A backward model selection was then used to remove insignificant covariates from the multivariable model. The variable with the largest P value was removed at each step, and the models with and without this variable were compared using the likelihood ratio test. If the P value of the likelihood ratio test was >0.05, the variable was removed from the model. Only variables with a P value <0.10 were kept in the final model.
Sensitivity analyses: generalized estimating equation and baseline MAP. Since the generalized estimating equation (GEE) method estimates the variance through a working correlation matrix using a robust variance estimate, we repeated the final model using GEE with an exchangeable working correlation structure as an initial sensitivity analysis. Because we could only control for baseline antihypertensive medications, changes in medications may have been a confounder in the longitudinal analysis. Therefore, we also did an analysis with only baseline (first) blood pressure data.
Replication sample. Finally, data from the African American Study of Kidney Disease and Hypertension (AASK) was used to confirm significant associations from the main analysis. The AASK study randomized African American men and women with hypertensive nephrosclerosis to treatment with ramipril, metoprolol, or amlodipine.15 Of the 1,094 subjects enrolled in the original study, 857 were enrolled in a follow-up genomics study. Linear regression analysis was used to determine the association between genotype and baseline MAP, controlling for covariates such as renal function, age, gender, and medications. A backward selection was used, and covariates with a P ≤ 0.1 were retained in the final model.
Results
Genotype associations with baseline parameters
Consistent with publically available databases, allele frequency differed by ancestry with T-allele frequencies of 0.50 and 0.16 in European and African Americans, respectively (Table 1; http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=705379). Those with a C/T genotype had the lowest high-density lipoprotein level (P = 0.008) and those with a T-allele had a higher prevalence of cardiac arrhythmia (P = 0.0008). The T-allele was also associated with a lower prevalence of hepatitis C (P = 0.0007), alcohol use (P = 0.005), and tobacco use (P = 0.004).
Longitudinal modeling of MAP
There were significant interactions between PON1-108 C/T (additive model) and MAP change between the younger and middle (P = 0.006), and between the younger and older (P = 0.003) age group (Table 2). Results suggested that the change in MAP from the younger to the middle or older age group was different depending on the number of C-alleles at PON1-108 C/T. In addition, a history of cardiac arrhythmia (P < 0.0001), hemoglobin A1C (P = 0.0005), method of recruitment (P = 0.002) and European American ancestry as compared with African American ancestry (P = 0.01) were also significantly associated with MAP change. A history of hepatitis C was marginally associated with MAP change (P = 0.06) and was retained in the final model.
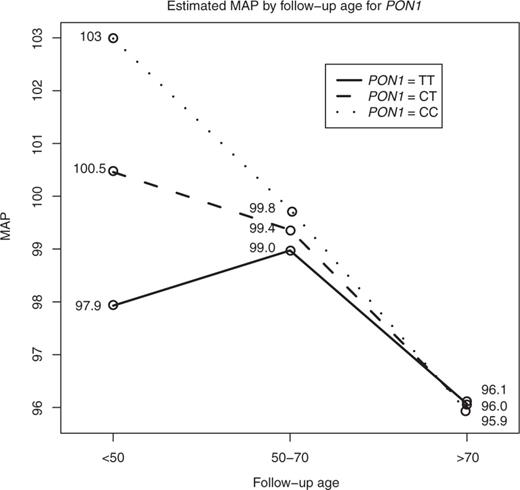
Because of the interaction between PON1-108 C/T genotypes and follow-up age, the data was first stratified by age in order to estimate the effect of PON1-108 C/T genotype on MAP (Figure 1). At a younger age, MAP increased by 2.53 mm Hg (95% confidence intervals (CI): 1.06, 4.00) with each additional C-allele at PON1-108 (Table 3). In the middle and older ages, MAP did not significantly vary by PON1-108 genotypes (Table 3).
Estimated mean arterial blood pressure (MAP) at follow-up age by PON1-108 genotypes. Estimated MAP from the random effects model with covariate adjustment by the three age groups is shown. There was a 2.53 (1.06, 4.00) increase in MAP with each additional C-allele at a younger adult (<50 years) age group. There was no significant difference in MAP by PON1-108 genotypes at older follow-up age groups. MAP also significantly declined from the middle (50–70 years) to older adult age groups (>70 years), and this decline was most pronounced for those with PON1-108 C/C genotype. PON1, Paraoxonase 1.
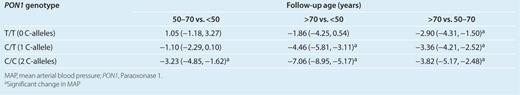
In order to estimate the effect of age on the change in MAP, the data was then stratified by PON1-108 genotypes (Table 4). The MAP at a middle age was significantly lower as compared with MAP at a younger age for individuals with two C-alleles. The estimated decrease in blood pressure was 3.23 mm Hg (95% CI: −4.85, −1.62). Significant decreases in MAP between older age and younger age were found for individuals with one or two C-alleles, with an estimated MAP decrease of 4.46 (95% CI: −5.58, −3.11) and 7.06 mm Hg (95% CI: −8.95, −5.17), respectively. Finally, there were significant decreases in blood pressure from middle to older age for all genotypes, with the greatest decrease among those with two C-alleles. Those with no C-alleles (T/T genotype) had a 2.90 mm Hg (95% CI: −4.31, −1.50) decrease, those with one C-allele (C/T genotype) had a 3.36 mm Hg (95% CI: −4.21, −2.52) decrease and those with two C-alleles had a 3.82 mm Hg (95% CI: −5.17, −2.48) decrease in MAP (Table 4 and Figure 1).
Sensitivity analyses: GEE and baseline blood pressure
Similar results were found using GEE (results not shown). Because changes in medications could have influenced blood pressure in the longitudinal analysis, the analysis was repeated with only baseline blood pressures. PON1-108 genotypes were significantly associated with MAP (P = 0.008; results not shown). In comparison with the younger age group, the interaction terms between genotype and follow-up age were marginally significant for the middle age group (P = 0.053) and for the older age group (P = 0.07; results not shown). Similar to the longitudinal results, there was a 4.0 mm Hg increase in MAP (95% CI: 1.04, 7.0) with an additional C-allele in the younger age group. There was no difference in MAP in the middle and older age groups by PON1-108 genotypes. Among those with two C-alleles, MAP significantly decreased between the older and younger age groups by 5.22 mm Hg (95% CI: −9.50, −0.93).
Replication sample
Of the 857 AASK study participants, 791 were genotyped at PON1 C/T-108. There was a significant interaction between genotype and median age (P = 0.02). For study participants below the median age of 54, there were significant differences in baseline MAP by PON1-108 genotypes, with higher MAP observed among those with a C-allele (P = 0.005). For study participants 54 years and older, baseline MAP did not significantly vary by PON1 genotypes (Figure 2).
Replication study from African American Study of Kidney Disease and Hypertension: distribution of baseline mean arterial blood pressure (MAP) by PON1-108 genotypes and age. In comparison to those with a C-allele, those with PON1-108 T/T genotype had a significantly lower baseline MAP among the younger (<54 years) study participants (P = 0.005). PON1, Paraoxonase 1.
Discussion
Main results
In this study, we used the longitudinal VAHC with blood pressure data on 1,302 hypertensive patients collected over an approximate 8-year period to explore the association between MAP and PON1-108 C/T, a promoter region polymorphism (rs705379). Results suggested that there was an estimated 2.53 mm Hg increase in MAP with each additional C-allele at a younger age (<50 years). There was also a notable decrease in MAP with age among those with C/C genotype. A sensitivity analysis using on baseline blood pressure yielded similar results. Finally, in an independent cohort of 791 African Americans with early hypertensive nephrosclerosis, the PON1-108 C-allele was also associated with higher baseline MAP among the younger participants <54 years.
PON1, cardiovascular disease and hypertension
Although PON1 genotypes have been most commonly studied in the context of association with atherosclerosis and cardiovascular disease,16 a large population based study suggested that PON1 activity was inversely associated with the risk of cardiovascular disease, but not independently of high-density lipoprotein cholesterol.17PON1 192 R has been associated with premature myocardial infarction,18 an 80% increased risk in coronary artery disease,19 number of diseased coronary vessels20 and an over twofold increase of stroke.21PON1 55 L is also associated with more advanced atherosclerosis.22
In this study, the PON1-108 C-allele was associated with higher blood pressure among those in the younger (<50 years) age group, suggesting that the C-allele is a determinant of early onset hypertension. This may be especially important among African Americans and other ancestral groups who have a higher frequency of this allele. However, this association is somewhat counter-intuitive because the C-allele has been shown to result in higher PON1 activity and is thought to be cardio-protective.8 Although we cannot be certain as to why we observed this association, the C-allele is also in linkage disequilibrium with L55M which has been associated with increased cardiac risk.8,20,22PON1-108 C/T genotypes were not significantly associated with MAP at older follow-up age groups consistent with research suggesting that PON1 activity decreases with age.23
At a population level, blood pressure is known to steadily increase with age, and then decrease somewhat with advanced age24. In this study, those with two C-alleles had a higher MAP at the younger age, with a clinically notable and significant decrease in MAP at middle and older age. At a population level one would expect an increase in blood pressure in the middle age group; however, it should be noted that how blood pressure changes with age and genetic stratification is unknown. In addition, comorbid disease and changes in medication could confound the longitudinal analysis. In order to address this, a sensitivity analysis using only baseline MAP yielded similar results for those with two C-alleles. In replication sample using AASK data, blood pressure did increase among those over the median age of 54 years. It should be noted that in comparison with VAHC, the AASK cohort had renal disease and was on average 10 years younger with a limited age range.
The association between PON1-108 C/T and MAP appears to be a somewhat novel finding. It is unknown whether PON1 modulates blood pressure independent of accelerated atherosclerosis or whether other family members such as PON2 located on smooth muscle cells may be contributing to blood pressure modulation. Although the C-allele has been shown to increase PON1 levels, PON1 is also up-regulated with the consumption of flavonoids, pomegranate, vitamin C, and the use of statins and down-regulated with high-fat diets, smoking and heavy alcohol consumption.25–27 Therefore, measured PON1 activity levels and the influence of environmental modulators should be considered in future studies. With respect to veteran patient populations, the effect of environmental toxin exposure during active duty may be an important consideration. Although there were significant associations between PON1-108 C/T and with comorbid disease, these associations did not remain significant after controlling for age and ancestry, suggesting that confounding as a result of population substructure was an important consideration in this dataset (results not shown).
Study strengths and limitations
Because of the longitudinal nature of this dataset, we were able to uncover a novel association between a PON1 polymorphism and blood pressure among the younger members of the VAHC cohort. In comparison with routine prepost blood pressure readings or measurements, we had a much more comprehensive sampling of outpatient measurements. Since changes in medications could have confounded our results, a sensitivity analysis with only baseline blood pressures was done, yielding similar results. Finally, we used both self-identified as well as genetically determined ancestry in our analyses to control for population stratification.
Although the VAHC is limited to study volunteers recruited from General Internal Medicine, this cohort appears to be representative to the general VA hypertension population.11 Residual confounding remains a concern especially in the study of complex medical traits. Whether selection or loss to follow-up biases the observed associations is unknown. This is also primarily a male veteran cohort, and whether these results can be extrapolated to other study populations remains to be studied. The limited number of Native Americans, Filipinos, and Latinos were grouped together; hence the findings here cannot be interpreted as specific to any one ancestral group. Finally, the effects of PON1 activity, diet and other environmental factors also remain to be explored.
Leveraging off the comprehensive VA electronic medical record system, we explored the association between PON1-108 C/T genotypes and longitudinal MAP over an approximate 8-year period. The C-allele at PON1-108 was associated with an estimated 2.53 mm Hg increase in MAP among younger adults (<50 years), with consistent findings in an independent cohort of African American men and women with hypertensive nephrosclerosis. This finding has both primary care and public health significance. PON1-108 genotypes may be an important determinant of hypertension among younger adults especially in ancestral patient populations such as African Americans who have a higher frequency of the PON1-108 C-allele. Study participants with the C-allele also had a notable decline in blood pressure with age, suggesting that careful attention to medication dosing may be important for these patients. Although it is premature to recommend genotype testing, further study of PON1 and family members PON2 and PON3 in larger multi-ethnic studies with younger adults is warranted in order to further understand the effects PON gene family variation on blood pressure.
The funding sources for this study are: National Institutes of Health (K23 RR020822, HL58120, EXPORT/CRCHD MD000220, GCRC RR000827, 1R01MH093500-01), Department of Veterans Affairs.
Disclosure
The authors declared no conflict of interest.
References
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.